Our Technology for Healthcare
Bioquell’s unique Hydrogen Peroxide Vapor technology is proven to eradicate bacteria, viruses, fungi, spores and more. The efficacy of our technology has been tested in numerous applications, in both controlled and clinical studies. The results showcase an unmatched level of effectiveness spanning every exposed surface of an enclosed area, providing leading infection prevention advantages for healthcare facilities around the globe.
Bioquell’s Hydrogen Peroxide Vapor Process
Achieve safe, fast, efficient, residue-free decontamination with Bioquell Hydrogen Peroxide Vapor in these easy steps.
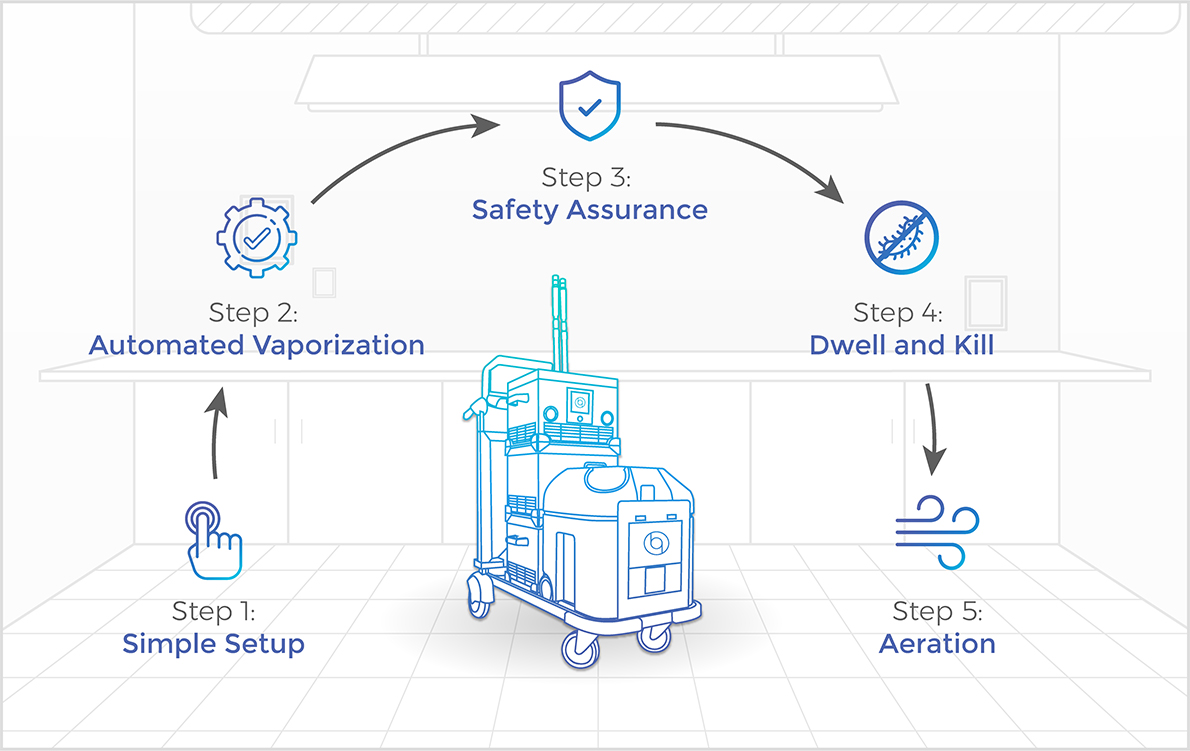
Step 1: Simple Setup
Quickly prep the room for decontamination: vents sealed, linens removed, and cabinets and drawers opened. After placing the system and aeration units in the room. Close and seal the door upon exiting.
Step 2: Automated Vaporization
Just press the green button to start the automated process. The Bioquell BQ-50 warms up and emits vapor onto every exposed surface, including in any adjacent bathrooms, sluices, store rooms etc.
Step 3: Safety Assurance
Once vaporization begins, the user checks the door to the room to ensure no leaks occur with the included handheld monitor. Leaks are rare as our process is not a pressurized one, and can be covered simply with Bioquell adhesive tape.
Step 4: Dwell and Kill
With vaporization now complete, the enclosed area is at a standstill, allowing the peroxide to dwell on every exposed surface but this stage is crucial if you want to ensure high efficacy against particularly tough organisms, like C. diff spores.
Step 5: Aeration
Aeration units kick on automatically to rapidly convert the Hydrogen Peroxide Vapor in to water vapor and oxygen, leaving no residue and no further cleanup required - once measured, the room is then safe for immediate patient reoccupation.
Bioquell Technology Efficacy
Bioquell’s Hydrogen Peroxide Vapor systems and services eliminate 99.9999% of pathogens for a 6-log kill with our 35% hydrogen peroxide solution. The process provides complete coverage on every exposed surface while remaining residue free, proven safe on sensitive electronics and shown to kill a wide range of microorganisms.
By using our Hydrogen Peroxide with a Bioquell system or service, you achieve complete surface decontamination. You can also ensure complete compliance to the laws and regulations within your country and other nations around the world. We are Biocidal Products Regulation compliant and EPA registered as a sterilant.
Download our Bioquell Efficacy Document for more than 50 references and peer-reviewed studies showcasing Bioquell’s efficacy in a quick-review format.