Bioquell Integrated Building Decontamination System
The Bioquell Integrated Building Decontamination System is a fully integrated solution designed for frequent or routine decontamination of the same area within a facility. This could be anything from a containment lab or multiple material air locks, to a sterile manufacturing core or biomedical holding area. Typically, Bioquell hydrogen peroxide vapour generator(s) are located centrally in a technical or unclassified space, and the vapour is distributed to the areas where it is required either through the building’s ventilation system or a dedicated network of pipes leading to nozzles on the ceiling of the target rooms. With an integrated system, no movement or storage of mobile equipment is required reducing manual setup times, increasing repeatability and removing human error.
Ideal for:
- GMP Biopharmaceutical manufacturing areas
- GMP Filling suites
- Biomedical facilities
- Biosafety/containment laboratories
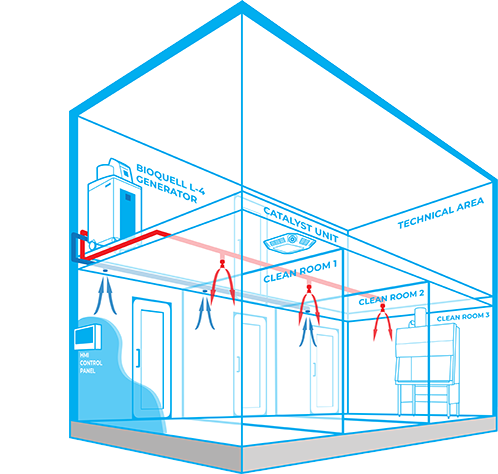
WHY CHOOSE THE Bioquell Integrated Building Decontamination System
Efficient
No mobile equipment to store or set up in classified area meaning no trailing cables or sealing of doors is required, removing human error

Effective Distribution
Vapour can be distributed either using the building’s ventilation system or through a dedicated network of pipes

Automated
Can integrate with BMS and utilise actuated valves to control the delivery of vapour into each individual room, allowing a number of different decontamination cycles to be run at the touch of a button
Rapid
Quicker setup times, faster decontamination cycles and the ability to run the system overnight means less downtime

Efficacious
Validated and programmed cycles ensure repeatable 6-log bioburden reduction every time

Safe
With the equipment located in an unclassified area, service and maintenance is easier and no personnel entry is required into a potentially contaminated target area
APPLICABLE SOLUTIONS
Validation
Following installation, every Integrated Building Decontamination System undergoes rigorous testing by our team of experienced validation engineers to ensure handshaking/communication to the building management system. Validation efforts ensue with the performance of an Installation and Operational Qualification (IQ/OQ) and customised 6-log decontamination cycles of any scale developed to the client’s specific needs. After testing and confirmed results, a fully functional decontamination system delivering repeatable results with high efficacy is available to use with the touch of a button.
Components

BIOQUELL INTEGRATED BUILDING DECONTAMINATION SYSTEM COMPONENTS




ACCESSORIES

Bioquell L-4
Use Bioquell’s versatile, multipurpose Hydrogen Peroxide Vapour generator to integrate into your facility in order to decontaminate areas on a consistent basis.

Use Bioquell’s versatile, multipurpose Hydrogen Peroxide Vapour generator to integrate into your facility in order to decontaminate areas on a consistent basis.

Bioquell’s proprietary high-purity aqueous 35% solution for use with the Bioquell L-4 system for 99.9999% deactivation of pathogens and a 6-log kill with full traceability and auditing through RFID label technology
Contact Us
To learn more about how Bioquell can fit your solution, please contact us.
UK Contact
Bioquell UK Ltd
52 Royce Cl,
Andover SP10 3TS, UK
+44 (0)1264 835 835
[email protected]










