Our Technology for Life Sciences
Combined, Bioquell’s decontamination systems and EPA-registered and BPR-compliant Hydrogen Peroxide Vapor provide consistent, GMP-compliant 6-log kills for all of your equipment and spaces. Biodecontamination is all that we do, so we ensure our solutions are safe for use with different materials in your varied environments for work that leads your field.
Bioquell's Hydrogen Peroxide Vapor Process
Achieve fast, efficient, residue-free decontamination with Bioquell Hydrogen Peroxide Vapor in four easy steps.
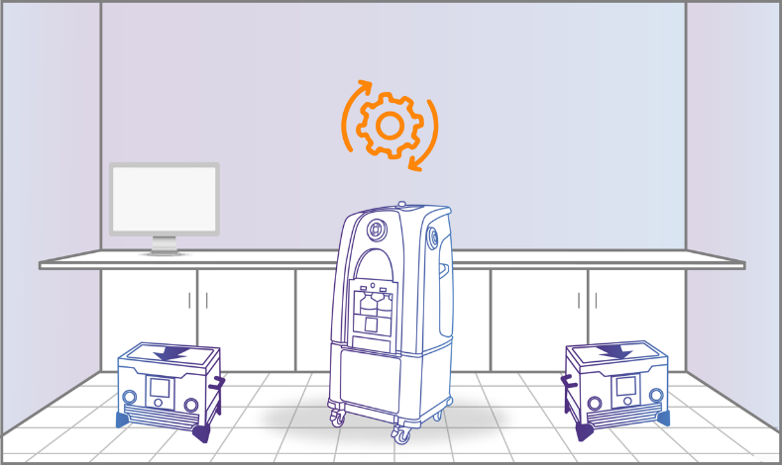
Step 1: Preparation
The Bioquell decontamination system warms up, getting ready to disperse the vapor. You do not need to reach or wait for temperature or humidity levels to begin.
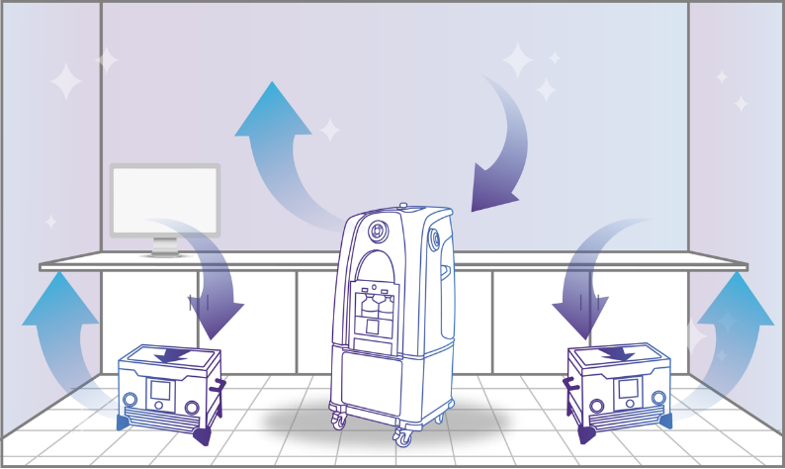
Step 2: Vaporization
The Bioquell decontamination system emits the vapor into the enclosed area and fills the space, pushing the vapor against every exposed surface, including surrounding complex shapes and crevices.
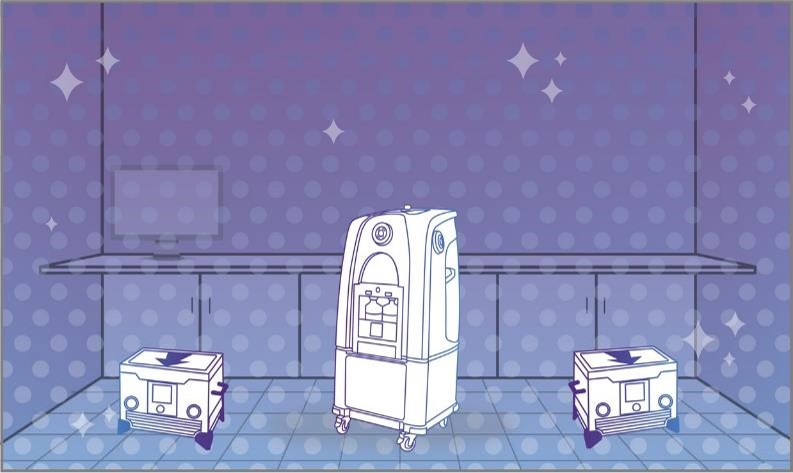
Step 3: Dwell
With vaporization complete, the enclosed area is at a standstill, allowing the peroxide to dwell on every exposed surface and kill pathogens.
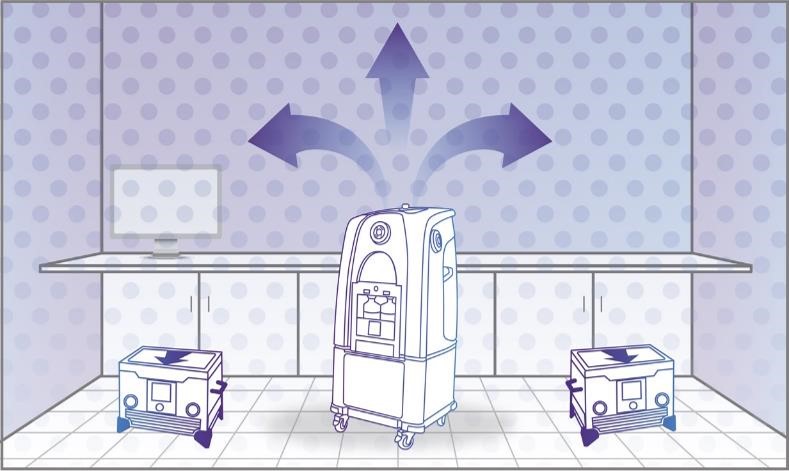
Step 4: Aeration
Your HVAC system, high-powered Bioquell aeration units using catalytic conversion or a combination of both now safely remove all of the Hydrogen Peroxide Vapor from the enclosed area. When aerated with Bioquell units, the vapor is converted into water vapor and oxygen.
Bioquell Technology Efficacy
Bioquell’s Hydrogen Peroxide Vapor systems and services eliminate 99.9999% of pathogens for a 6-log kill with our 35% hydrogen peroxide solution. Through a complete coverage of every exposed surface in an enclosed area, the process is residue free, proven safe on sensitive electronics and shown to kill a wide range of microorganisms including bacteria, viruses, fungi, spores and more.
By using our Hydrogen Peroxide with a Bioquell system or service, you achieve even more than complete surface decontamination. You can also ensure complete compliance to the laws and regulations within your country and most others around the world. We have built our business around one goal: to be the decontamination solution for any company from a start-up enterprise to a global corporation.
Detailed reports and data support the efficacy of Bioquell’s Hydrogen Peroxide Vapor technology. They range from our EPA sterilant registration and materials compatibility to European Biocidal Products Registration (BPR) compliance to numerous studies.
Download our Bioquell Efficacy Document for more than 50 references and peer-reviewed studies showcasing Bioquell’s efficacy in a quick-review format.