
Ensure Business Continuity with Bioquell
With Bioquell Rapid Bio Decontamination Service (RBDS) service options, your company can be prepared to shut down a contamination event quickly. The risk of contamination is a major concern in pharmaceutical and particularly biologics manufacturing. The impact of a contamination event can be huge both in terms of costs, lost revenue and drug shortages for patients. Be ready with Bioquell.
Watch Video 
Learn More About Bioquell RBDS and Business Continuity Offerings
Building Bioquell RBDS into business continuity plans is a proactive approach to limit the impact of a contamination event and minimize the extent of any downtime. RBDS can be incorporated into these procedures in several ways to increase security.
PRIORITY RESPONSE PLAN (PRP)

Be proactive with the PRP. We’ll work together to understand your specific facility requirements and then create a detailed plan of how the decontamination process is written and will be activated in the event of a contamination.
Benefits include:
- Bioquell will be an approved contractor
- Reduced deployment time
- Reduced costs compared to standard decontamination deployment
CRITICAL RESPONSE PLAN (CRP)

Get faster decontamination response time with all the benefits of a PRP. Dedicated, specialized equipment is housed onsite or at the nearest Bioquell facility and is checked monthly to ensure it’s fully operational and ready when needed.
Benefits include:
- Response times of 24, 36 or 48 hours
- Reduced deployment times
- Decrease in lost production time

A Simple Process
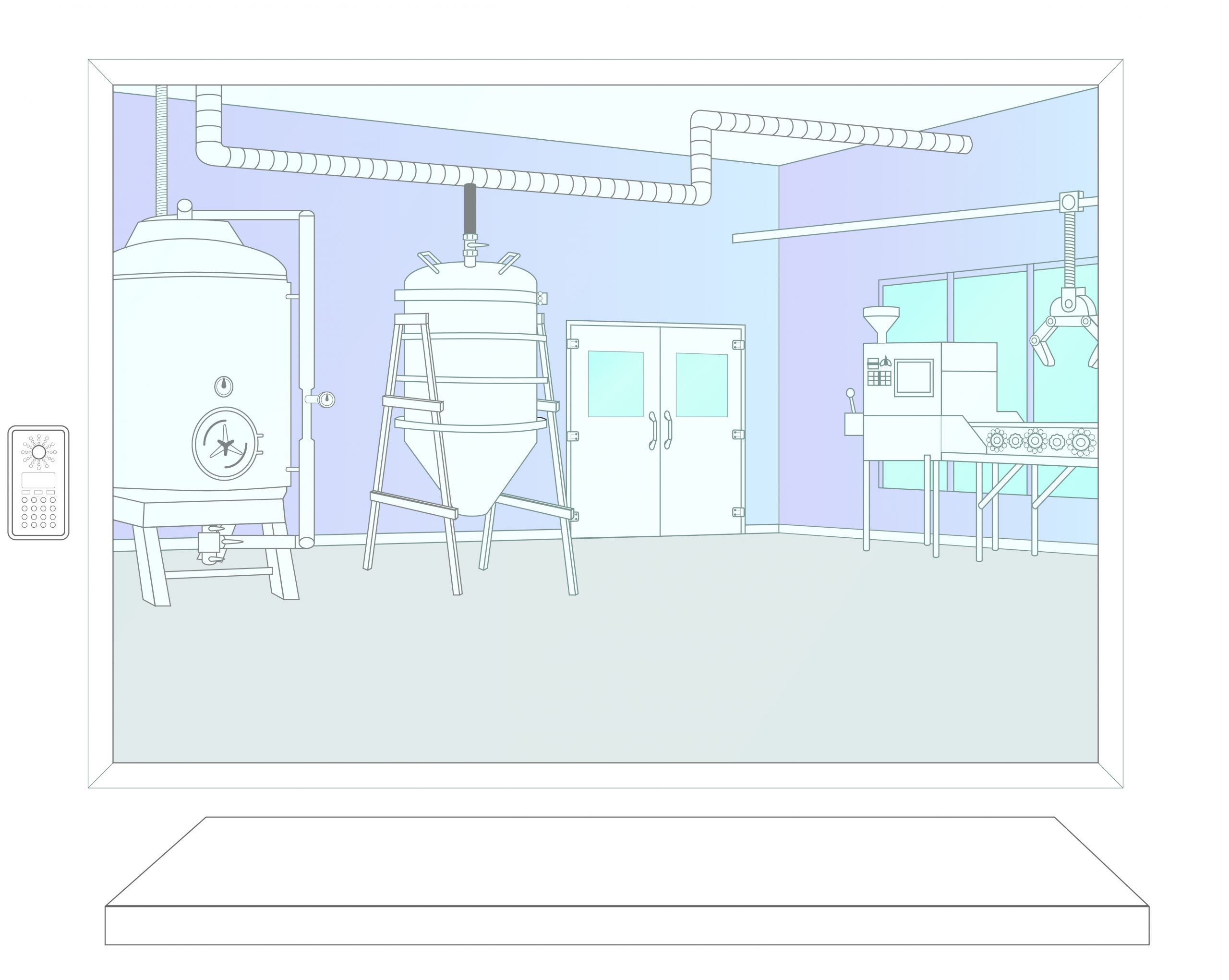
Step 1: Activation
In the event of a contamination the Bioquell RBDS team is activated. Equipment and engineers are dispatched to the site. Site personnel prepare the area following the established response plan.
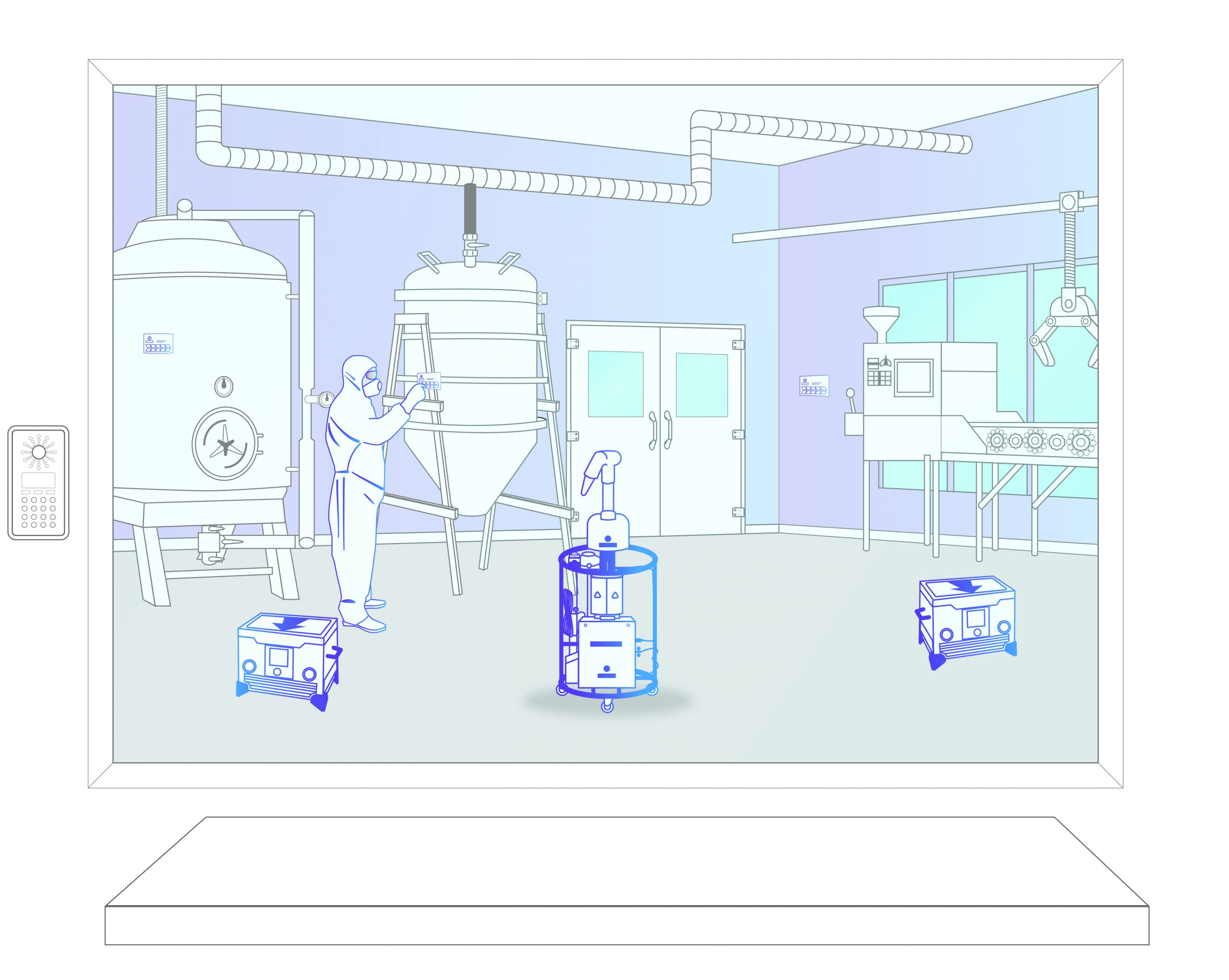
Step 2: Equipment Setup
The Bioquell team brings equipment on to site in line with client SOPs. The equipment, biological and chemical indicators are placed in the positions defined by the response plan.
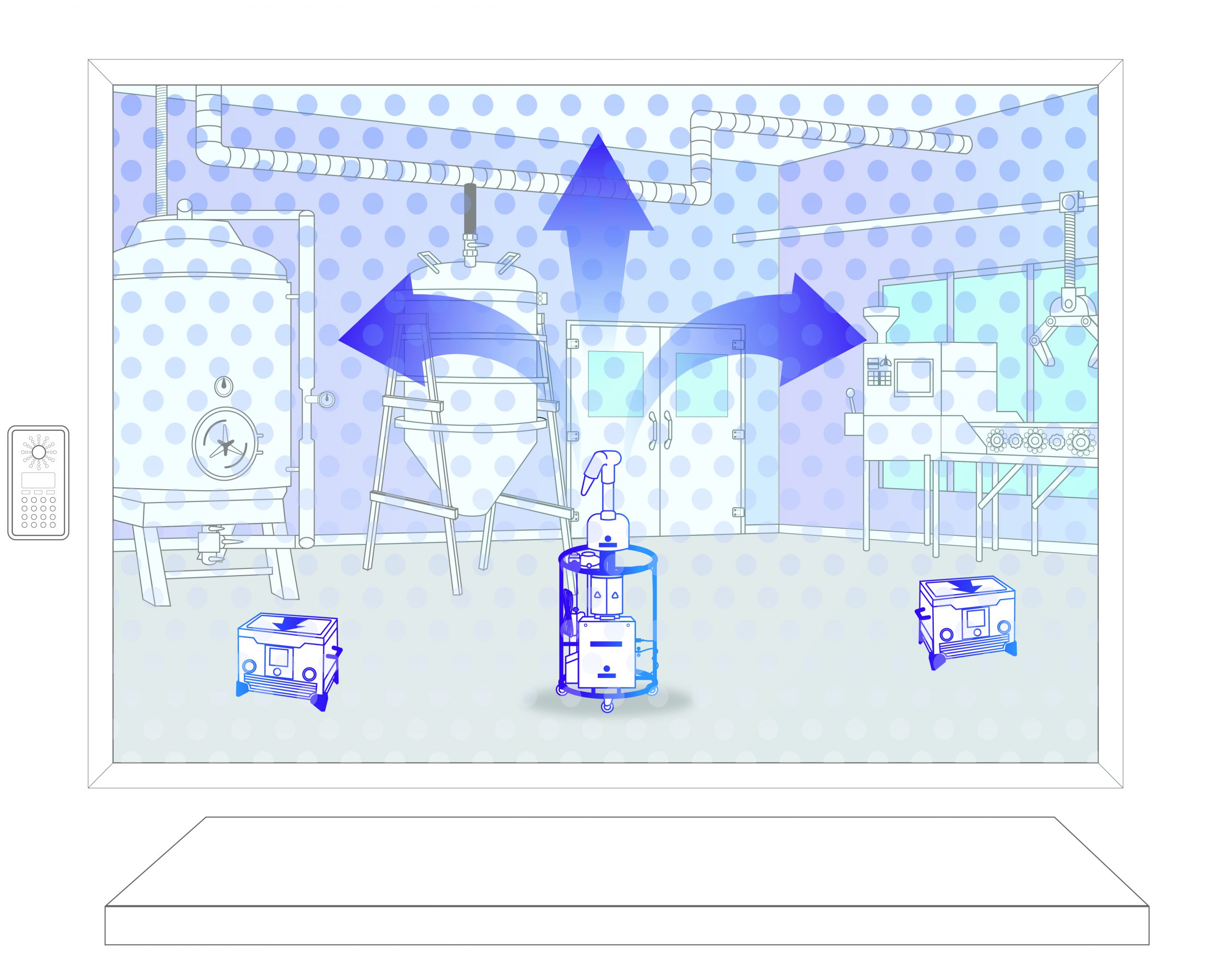
Step 3: Decontamination
Vapor is injected to to achieve a 6-log sporicidal kill. After this, it is safely broken down into oxygen and water.
Step 4: Recommissioning
Equipment removal, handover of the area and delivery of a full report - the service is fully documented, fully managed and verified using BIs.


RBDS in Action
See how the Bioquell RBDS process works in this short walkthrough video.
Contact Us
To learn more about how Bioquell can fit your solution, please contact us.
The Americas
Bioquell Inc.
702 Electronic Dr. Suite 200
Horsham, PA 19044
+1 215 682 0225
[email protected]







